Something that fascinates me about the science of global warming is that some climate ‘experts’ do not have any better understanding of the basic physical processes involved than the lay person does.
For instance, in a recent paper of mine that was rejected for publication in Geophysical Research Letters, the editor assigned only a single peer reviewer, who did not even understand what causes temperature to change. And yet, the reviewer was casting judgment on my analysis that showed that the cloud-altering behavior of the Pacific Decadal Oscillation over the last 100 years might explain most of what we now call climate change and global warming.
So, let’s review the basics of why the temperature of an object changes. In a few minutes, you will have a better understanding than even that (presumed) climate expert has. It doesn’t matter whether it is the Earth, or a pot of water on the stove, the fundamental concepts are the same. And if you think you already understand why a pot of water on the stove warms up, you might be in for a surprise.
Question: TRUE or FALSE? A pot of water on the stove warms because the stove is transmitting heat into the pot.
Answer: FALSE.
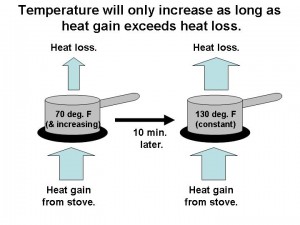
Well…it’s only half true. Let’s examine what happens when we place a pot of room temperature water on a hot stove. In the following figure I’ve illustrated the flows of heat with arrows.
Let’s say it is a gas stove, so that we can produce a small, constant flame under a pot of room temperature water, with no lid. Heat flows from the stove into the pot, and the water gradually warms.
After about 10 minutes, though, the water reaches a certain temperature and just stays there. But how can this be? How can the stove still be pumping heat into the pot, and yet the water has stopped warming? Obviously, what controls the temperature of the water is more than just the rate of heat input from the stove.
What we are missing is that the pot was losing heat to its surroundings at the same time it was gaining heat from the stove. As illustrated in the above figure, the temperature of the pot will only change if there is an imbalance between the rate of heat flow into the pot and the rate of heat flow out of the pot.
As the water started to warm on the stove, the pot became warmer than its surroundings, and so it also started to lose heat to its surroundings. This happens through three processes: (1) faster evaporation of the water, (2) air currents flowing past the pot and picking up heat and carrying it away, and (3) loss of infrared (heat) radiation of the warmer pot to its cooler surroundings.
As the water got warmer and warmer, those three heat loss processes accelerated. Finally, a temperature was reached where the rate of heat loss by the pot equaled the rate of heat gain from the stove. It is only at this point of energy balance that the temperature stops changing.
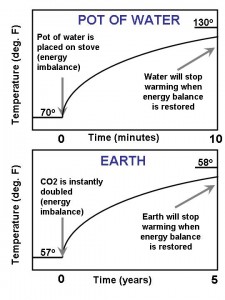
And, as I have illustrated in the following figure, it doesn’t matter whether it is a pot of water or the Earth, the basic concept is the same. An imbalance in the rate of energy flows in and out of the Earth, or the pot, will lead to a temperature change.
The main difference is the length of time involved for the temperature to respond to an imbalance: It takes only minutes for a few inches of water in a pot to come to a new equilibrium temperature. But it can take years for an energy imbalance of the Earth to cause hundreds or thousands of meters of ocean depth to fully respond with a temperature change.
Also note that warming can be caused either by (1) increasing the rate of energy gained, OR (2) decreasing the rate of energy lost. It is the second process that is involved in the theory of manmade global warming: Increasing atmospheric CO2 concentrations slightly reduce the rate at which infrared heat is lost by the Earth to outer space, leading to an energy imbalance. Warming then results, which stops after energy balance is once again restored as a warmer Earth radiates infrared energy at a greater rate to outer space.
In fact, the mechanism of heat loss by the Earth is simpler than that of a pot of water on the stove. Because there is no air in outer space to carry heat away from the Earth, there is only one heat loss mechanism, not three: Infrared radiation.
Of course, anything that can change the rate of energy input or output can cause climate change. For instance, natural fluctuations in atmospheric circulation patterns can alter global cloud cover by a small amount, thereby changing how much sunlight is allowed to reach the Earth’s surface. Or, circulation changes might result in small wind shear-induced changes in precipitation efficiency, thereby altering how much water vapor – our main greenhouse gas – is allowed to remain in the atmosphere. These natural fluctuations can cause warming or cooling.
And these are only two examples of the many potential kinds of natural climate change that the Intergovernmental Panel on Climate Change (IPCC) simply assumes do not exist.

 Home/Blog
Home/Blog